Many of us have been taught a simple story: eat too much, gain weight, and eventually develop insulin resistance that may lead to type 2 diabetes. But what if this narrative isn't just oversimplified—it's fundamentally flawed? As we dig deeper into metabolic health, we're discovering that the relationship between insulin resistance and obesity is far more complex and bidirectional than previously understood.
The Conventional Wisdom Gets It Backward
For decades, the medical establishment promoted the idea that obesity causes insulin resistance. The story seemed intuitive: excess body fat, particularly visceral fat surrounding our organs, creates inflammation and disrupts normal insulin signaling.
However, compelling evidence suggests we may have been viewing this relationship through the wrong end of the telescope. What if insulin resistance isn't just the consequence of obesity, but often its primary driver?
When we develop insulin resistance, our pancreas compensates by producing more insulin to maintain normal blood glucose levels. This elevated insulin—hyperinsulinemia—is problematic because insulin is fundamentally an anabolic hormone that promotes fat storage and blocks fat oxidation. In other words, high insulin levels make it nearly impossible for your body to access and burn stored fat, creating what I call a "fat-trapping" scenario.
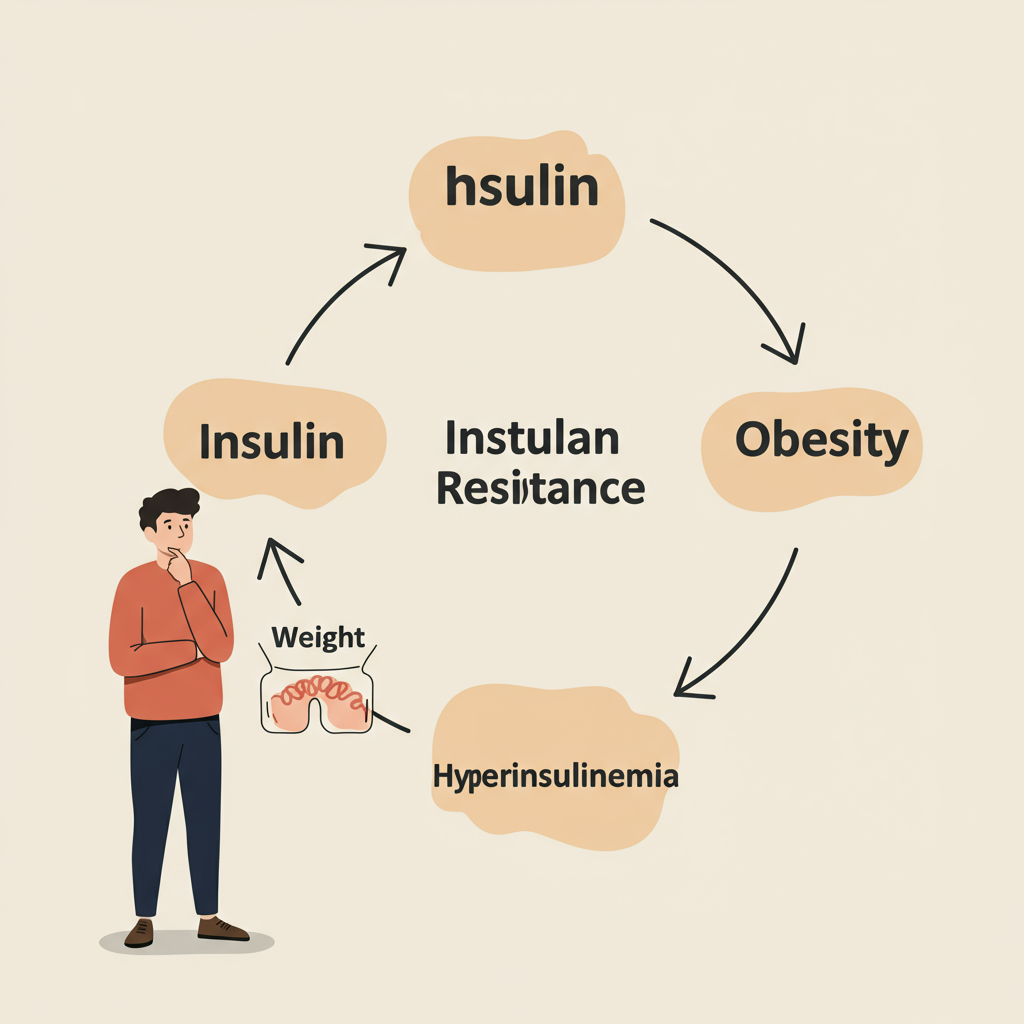
The Hyperinsulinemia Trap
Here's what happens in this metabolic trap:
- Initial insulin resistance develops (often from excessive consumption of refined carbohydrates and sugars)
- Pancreas increases insulin production to compensate
- Chronically elevated insulin levels make it nearly impossible to access stored fat for energy
- The body perceives an energy crisis despite having abundant stored energy
- Hunger signals increase, driving greater food consumption
- Weight gain occurs, which can further worsen insulin resistance

This creates a vicious cycle. The more insulin resistant you become, the more insulin your body produces, making it increasingly difficult to burn fat and increasingly easy to store it. This isn't just about willpower or calories—it's about hormonal signaling gone awry.
In many people, hyperinsulinemia precedes significant weight gain. This explains why some individuals with normal BMI can still have pronounced insulin resistance (sometimes called "metabolically obese normal weight"), while others with obesity can maintain relatively normal insulin sensitivity.
Breaking the Cycle Through Metabolic Intervention
Understanding this bidirectional relationship changes how we approach treatment. Simply telling someone with hyperinsulinemia to "eat less and move more" without addressing the underlying insulin resistance is like telling someone with a broken leg to run faster.
More effective approaches include:
Dietary modifications:
- Reducing refined carbohydrates and added sugars
- Prioritizing protein and healthy fats
- Considering time-restricted feeding to improve insulin sensitivity
Targeted exercise:
- Incorporating resistance training to build muscle (a major site of glucose disposal)
- Adding zone 2 cardio to improve mitochondrial function and insulin sensitivity
Medication (when appropriate):
- GLP-1 agonists not only reduce appetite but can improve insulin sensitivity
- Metformin reduces hepatic glucose production and can lower insulin levels
The critical insight is that we must break the hyperinsulinemia cycle to enable sustainable fat loss. When insulin levels decrease, the body can finally access stored energy, reducing inappropriate hunger signals and enabling fat oxidation.

Rethinking Our Approach to Metabolic Health
This bidirectional relationship between insulin resistance and obesity demands we reconsider our approach to metabolic health. Rather than focusing exclusively on weight loss as the primary goal, we should target the underlying metabolic dysfunction—specifically insulin resistance.
By improving insulin sensitivity first, weight management often becomes significantly more achievable. This represents a paradigm shift from the "calories in, calories out" model to a more nuanced hormonal model of obesity.
For many struggling with weight management, this perspective offers hope. Their challenges may not stem from lack of discipline but from a fundamental metabolic dysfunction that can be addressed through targeted interventions.
The path forward requires personalized approaches that recognize the complex, bidirectional relationship between insulin resistance and obesity. By breaking the hyperinsulinemia cycle, we can unlock not just weight loss, but genuine metabolic health.
References:
Ludwig DS, Ebbeling CB. The Carbohydrate-Insulin Model of Obesity: Beyond "Calories In, Calories Out". JAMA Intern Med. 2018;178(8):1098–1103.
Corkey BE. Diabetes: Have We Got It All Wrong? Insulin hypersecretion and food additives: cause of obesity and diabetes? Diabetes Care. 2012;35(12):2432-2437.






