Introduction
Continuous Glucose Monitoring (CGM) devices have evolved significantly in their design and user experience, making them more convenient and reliable. This article focuses on four critical aspects of CGM design and usage: signal transmission methods, insertion mechanisms, adhesive considerations, and placement guidelines.
1. Signal Transmission Methods
CGMs use different methods to transmit data, with two main types in the market today:
-
NFC Transmission (Near Field Communication):
NFC-based CGMs require manual scanning with a reader or smartphone to upload glucose data. This can be cumbersome for users, as it involves repeated manual efforts and often leads to missed data points due to scanning gaps. -
Bluetooth Transmission:
Modern CGMs, including the Sus-Wel Linx, utilize Bluetooth for seamless data transmission. This method offers continuous data flow, eliminating the need for manual scans and providing a more user-friendly experience. To ensure consistent connectivity, users must keep their phone’s Bluetooth enabled. If connection issues arise, restarting the CGM app can help reestablish the link.
2. Insertion Method

CGM sensors are designed for comfort and minimal invasiveness. Here’s how the insertion process works:
- The sensor involves a fine, flexible filament that is inserted into the dermis (a layer of the skin) using an insertion needle. This needle is temporarily used to guide the sensor and is then retracted, leaving only the sensor filament in place. While the insertion needle may look intimidating, it does not remain in the body and is part of a safe, well-designed process.
- Importantly, in a survey, 95% of users reported that sensor insertion was pain-free or only mildly uncomfortable, underscoring the minimally invasive nature of CGM devices.
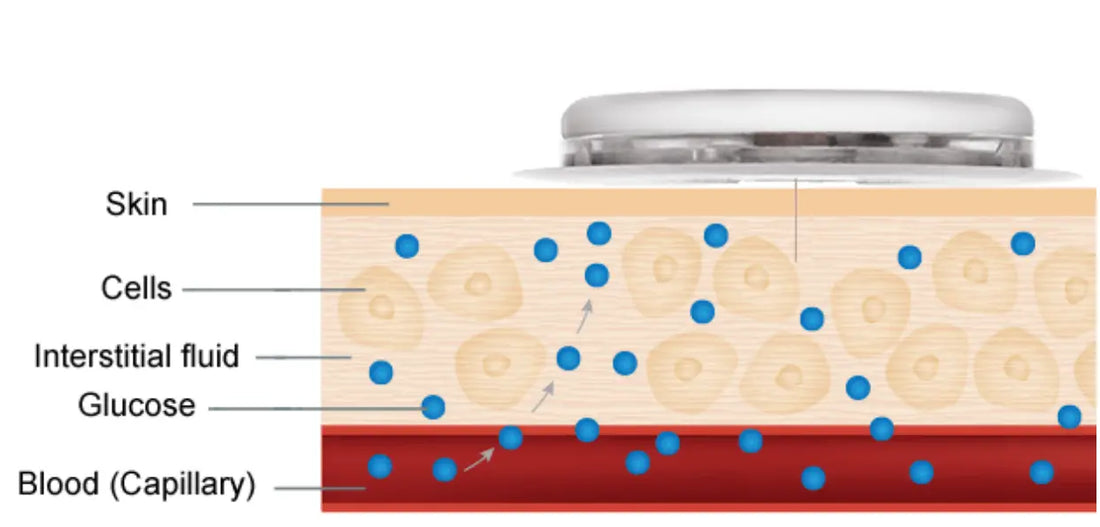
3. Placement Location
Proper sensor placement is essential for accurate readings and user comfort. CGMs, including the Sus-Wel Linx, have been tested and cleared to be worn on the back of the upper arm for up to 15 days. This location ensures optimal contact with interstitial fluid, stable data transmission, and user comfort over extended wear periods.
4. Adhesive Considerations
The adhesive used to secure CGMs to the skin can be a source of concern for some users due to potential allergic reactions. Traditional CGM adhesives sometimes contain compounds like IBOA (isobornyl acrylate) and MBPA (methyl-bisphenol A), both of which are used in manufacturing medical adhesives and plastics and may trigger allergies in sensitive individuals.
- Sus-Wel Linx CGM Adhesive: The adhesive used in the Sus-Wel Linx CGM does not contain IBOA or MBPA, reducing the risk of allergic reactions and offering a safer, more comfortable experience for users.
Conclusion
By understanding the design and usage details of CGMs, users can maximize their benefits and feel more confident in their glucose management journey. The Sus-Wel Linx CGM’s use of Bluetooth transmission, comfortable insertion process, adhesives free of IBOA and MBPA, and convenient placement options make it a leader in modern CGM solutions.